Alternate donor transplantation
What is HLA matching? What is its implication in stem cell transplantation?
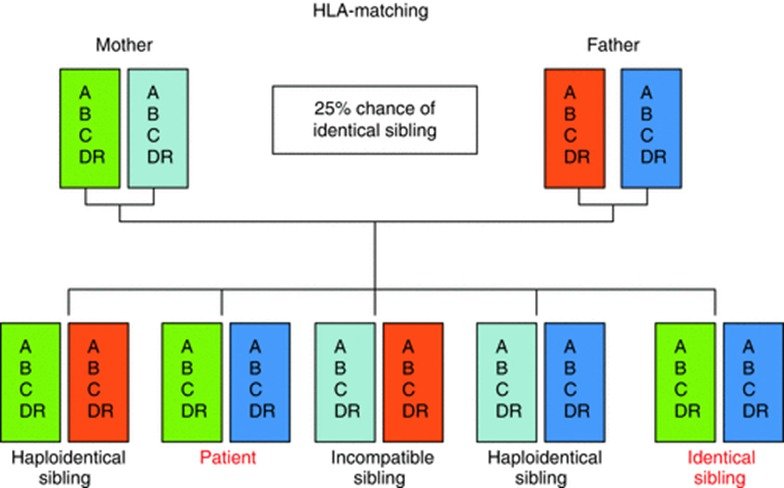
The Human leucocyte antigen genes code for proteins which are present on surface of most of the cells in our body. They form the basis of how our body’s immune system recognizes ‘self’ from ‘non-self’. Matching for these genes is a pre-requisite before going ahead with a stem cell transplant to prevent ‘rejection’ of the stem cells from a donor. This is done through a blood test where HLA genes of a potential donor is matched against the genes of patient. As we get 50% of our genes from each of our parents, there is a 25% chance of a person being a complete match with a sibling – the more the number of siblings, the higher the chance of finding a complete HLA match within the family (Fig. 1). Such a transplant is called Matched Sibling Donor (MSD) transplant. Only 30% of our patients manage to find a fully matched sibling donor. For the rest of our patients, we need to resort to matched unrelated donor (MUD) or Haplo-identical stem cell transplants.
What is the difficulty with MUD and Haplo-identical transplant?
The degree of HLA matching ensures that the stem cells that are introduced into the patient are not ‘rejected’ by the immune system of the patient. The vice-versa is also true i.e., the risk of graft versus host disease (GVHD – donor cells acting against host tissues) is also dependent on the degree of HLA match. So, a fully matched sibling donor transplant requires the least immune suppression to prevent graft rejection and GVHD. In contrast, for a Haplo-identical transplant the chances of both of these complications is very high and hence potent immunosuppression is needed. This in turn places the patient at high risk of opportunistic infections.
When are MUD and Haplo-identical transplants performed?
As already mentioned, only 30% of our patients have a matched sibling donor available. For the rest 70% requiring stem cell transplantations, MUD and Haplo-transplants are the way forward. A donor search is initiated for a patient who doesn’t have a sibling match. There are stem cell registries available for this. If a match is found, the donor is informed and his stem cells are collected and the shipped to the site of transplant. Sometimes, despite search a matched transplant donor is not found. The choice then is to find a donor with next best match in the family. Out of the HLA loci that are usually checked for (HLA- A, B, C, DR, DQ), the haplo-identical donor with the maximum match is selected as a potential donor
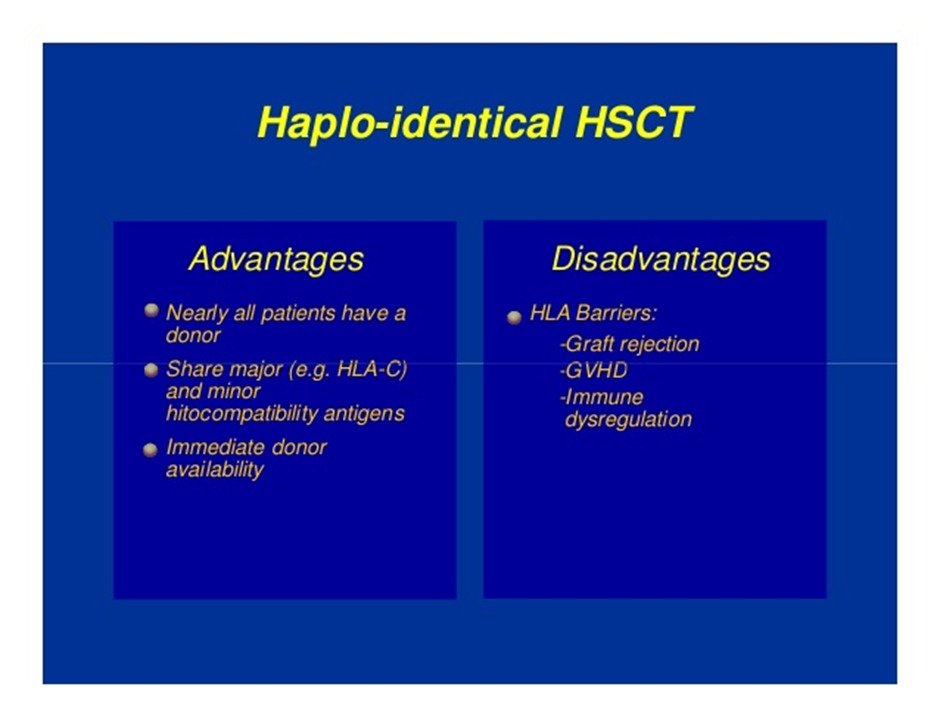
How are haplo-identical transplants different from MSD-transplants?
1. They are technically more demanding due to usage of high immunosuppression and handling side-effects because of it.
2. The degree of supportive care, duration of hospital stay, follow-up is higher. This means more financial burden too.
3. The usage of such transplant is increasing as more families are becoming nuclear and chance of a matched sibling donor is decreasing further.
Principles of Hematopoietic stem cell transplantation
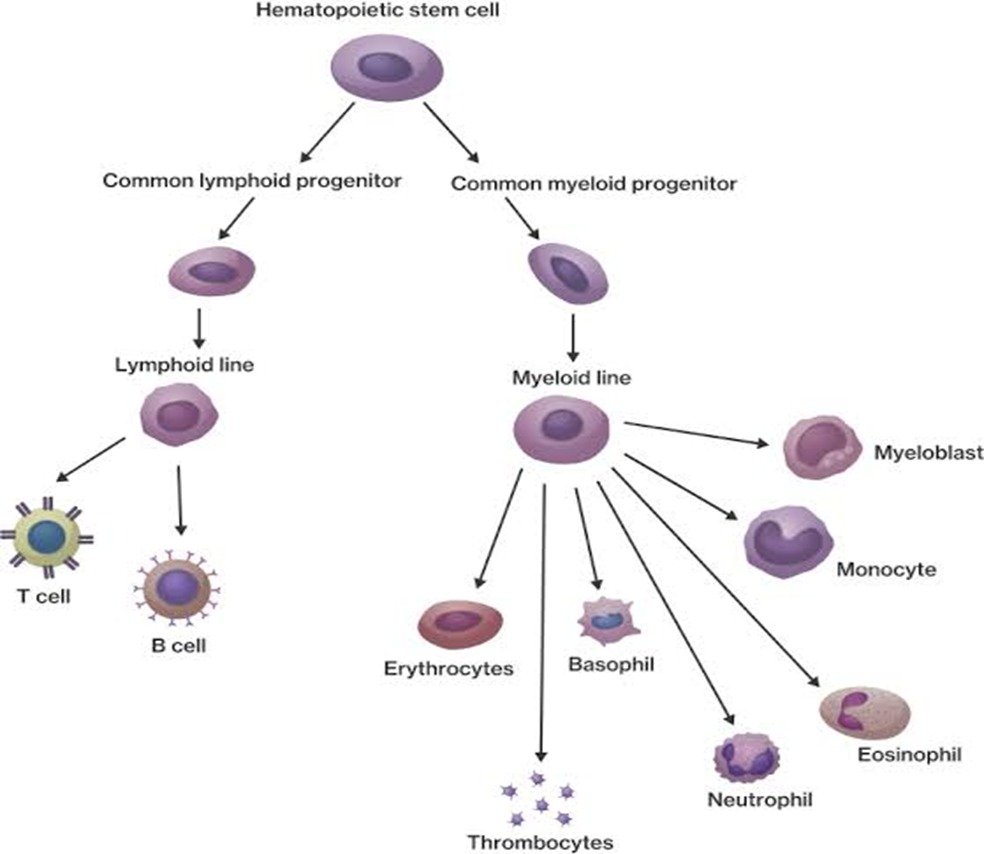
Hematopoietic stem cells are progenitor cells that possess the capacity for self-renewal and multi-lineage differentiation. They reside predominantly in the bone marrow and eventually give rise to terminally differentiated blood cells e.g., red cells, white cells and platelets. In contrast to these stem cells, the terminally differentiated cells have a relatively short life span and need to be replenished by the bone marrow.
Hematopoietic stem cell transplantation has the potential to cure patients with relapsed or high-risk hematological malignancies as well as patients with inherited and acquired disorders of the hematopoietic and immune systems that may be incurable with conventional therapy. The goal is to replace the recipient`s defective hematopoietic and immune systems with normal hematopoietic stem cells from a ‘closely matched’ donor. The eradication, treatment, and possible cure of these malignant diseases at times may need doses of chemotherapy or chemoradiation therapy that exceed the tolerance of the bone marrow. Infusion of hematopoietic stem cells rescues potentially fatal, irreversible marrow aplasia and allows administration of high dose chemotherapy or chemoradiation therapy to treat hematologic cancers.
Stem cell transplantation can be divided into autologous (auto-) HSCT or allogeneic (allo-) HSCT basing upon what the source of stem cell is. Inautologous HSCT, in which the patient`s own bone marrow is the source of hematopoietic stem cells. In Allogeneic HSCT, an HLA-matched donor isthe source of stem cells. These are administered following either a reduced intensity or myeloablative (so- called full intensity) regimen. The donor graft is obtained from an HLA- matched or HLA mismatched family member (usually sibling) or an unrelated donor such as an adult matched unrelated donor (MUD) or an umbilical cord blood graft. Recently, the use of haploidentical family member donors had been an emerging graft source.
Broadly, hematopoietic stem cell transplantation consists of three parts: a conditioning regimen – where high dose chemotherapy (and sometimes radiotherapy) is used to eradicate residual cancer cells; stem cell infusion – where stem cells help in production of normal blood cells; prophylaxis of graft- versus-host disease(GVHD) – a unique condition where stem cells from donor react against recipient’s organs; supportive care – of treating complications of HSCT. Prophylaxis for and treatment of GVHD is required in case of allogeneic transplants only, which can be achieved through immunosuppressive medications or manipulating the graft (particularly T cell depletion).The choice of conditioning regimen, stem cell source and GVHD prophylaxis regimens varies on the basis of patient and disease characteristics, as well as donor availability. An intensive supportive care is required to tide over long duration of low blood counts; bacterial, viral and fungal infections; micro-vascular complications; transfusion support with blood and platelets etc.
Advances in HLA typing, supportive care and newer immunosuppressive agents have significantly improved the long term survival rates after hematopoietic stem cell transplantation over the past 10 years. These patients require a long period of convalescence to return to a normal immune system, requiring a close surveillance for infections, recurrence of GVHD, repeat vaccinations etc.

